Chemical Industry Update: Regulatory Compliance Requires a Single Source of Truth
Many industry sectors look to the chemical industry as an exemplar for driving key product advancements. The industry’s contributions to other industries span the world, but there is continued pressure for them to improve their supply chains. The United Nations Conference on Environment and Development in 1992, also referred to as the “Earth Summit,” initiated the movement toward “eco-efficiency” in the handling and disposal of toxic chemicals, building worldwide momentum for governments and corporations to carefully reexamine their regulations and policies regarding chemicals in the global supply chain.
There is a concurrent opportunity for chemical industry executives to respond to the increase in supply chain environ- mental regulations in an evolving global economy by seizing competitive opportunities. Companies are looking for ways to centralize, integrate, automate and optimize applications across multiple locations while allowing for a tremendous amount of flexibility and variability in labeling. In addition, efficiency, cost savings, accuracy, and reliability in labeling can benefit businesses in today’s regulatory environment like never before. Enterprise labeling is the intelligent solution.
Label Demands and Risk Management
Every industry has product-labeling challenges, but the chemical industry stands out as a sector with extraordinary risk management issues. From raw materials and manufacturing facility requirements to end-user delivery, every step of a chemical product’s passage through the supply chain must be understood, strategized and implemented without failure. For this sector in particular, mistakes can be disastrous.
At the same time, the chemical industry also faces many of the same issues associated with less dangerous or toxic substances: counterfeiting, diversion, requirements for authentication and a maze of regulations and mandates. Complex supplier/partner relationships and multi-layered supply chain models increasingly characterize the industry. With the emergence of global data and labeling standards, it is clear that the chemical industry must advance its traditional supply chain and product labeling models to accommodate the new realities of label harmonization.
More chemical industry executives are looking for a “single source of truth” in a centralized and secured labeling system. Reduction of the amount of labels and applications while supporting the variability of the regulatory, customer, industry, and product demands is a top priority.
GHS, REACH, and OSHA: The Real Implications
When an industry standards organization or community proposes a voluntary date of compliance, industries can operate in a more flexible atmosphere. On the other hand, when government regulatory bodies with strong enforcement capabilities mandate new processes and procedures, industries must comply or face strict consequences. Global readiness for evolving industry standards includes operating according to the Global Harmonized System of Classification and Labeling of Chemicals (GHS); Registration, Evaluation, Authorization and Restriction of Chemical Substances (REACH); and the regulatory Occupational Safety and Health Administration (OSHA) in the U.S., which has recently aligned with GHS. Product labeling regulations mandated by governments can mean the difference between market entry and exclusion. Global and regional standards and regulatory compliance has become a basic necessity for any chemicals manufacturer.
The deadline of June 1, 2015 for the United States OSHA Hazard Communication Standard (OSHA HCS), which was modified in 2012 to meet the Globally Harmonized System (GHS), is pushing hard on the heels of all U.S. manufacturers for handling, shipping, and receiving products containing hazardous chemical ingredients. A total of sixty-seven countries including the U.S. have opted to meet the GHS standard with the goals of gaining a competitive edge, and to strive for increased supply chain efficiency. To meet OSHA’s HCS requirements and align with GHS, labels must include a product identifier, manufacturer’s contact information, signal word, pictogram, hazard statement, and a precautionary statement for each hazard class and category. By June 1, 2016, to avoid fines and penalties, U.S. companies must be fully compliant with OSHA’s updated regulations.
In the European Union (EU), the Classification, Labelling and Packaging Regulation (CLP) has also been aligned to GHS as well. Failure to label according to the new rules does not simply result in a sternly worded letter suggesting that an organization improve its practices; it results in significant regulatory barriers to ongoing business operations. Fines will be imposed. Product shipments will be halted. Recalls will be ordered.
Failure to comply means ongoing sales are affected, customers look for alternative sources, investors seek more secure opportunities, and competitors seize the opportunity to reposition themselves. This kind of regulatory exposure is bad for business, to say the least.
Correct Label Content is Good Business
There are two significant ways labeling can go awry. First, a label with the correct product information can accidently be applied to the wrong product. The second occurs when a label is applied to the correct product, but the label contains inaccurate or incomplete content. Ideally, of course, products with the correct labels and required content go to the right places, customers get the product they ordered, and these products speed through the supply chain and reach their intended destination with less delay. Late delivery penalties are averted. Plus, the potential for re-identifying and re-labeling distribution center product stockpiles is diminished, saving time and cost to operations. It becomes apparent that understanding and managing correct content labeling is an essential part of a successful business.
Failure to manage labeling at an enterprise level with agility can jeopardize corporate sustainability at its very core. How do labeling errors like these occur? Several major factors cause labeling inefficiencies and errors that can impact corporate revenue and market share, while attacking these risks head-on can win a significant competitive corporate advantage. Risks include:
Mislabeling
The wrong label or a label with incorrect or incomplete data typically misdirects the product, often at distribution centers, until the problem can be identified and corrected. Inventory carrying costs can soar.
How are labels with inaccurate content created?
There are several identified internal culprits. First, the organization does not have an enterprise-wide and centrally controlled system for label and content generation in support of every location where labels are applied. This means different plants— even different manufacturing lines—can produce labels locally to a different standard and with different sources of label content. This is further aggravated when individuals at a remote manufacturing facility are not experienced with labeling systems, technology, regulatory compliance issues, and/or lack on-site IT support for labeling. This has led many organizations to go to extraordinary measures to overcome the problem, but not in ways that optimize their business or supply chain.
For example, one manufacturer facing this problem shipped all of its products made in Asia to the company’s headquarters in Georgia, USA where experienced professionals applied labels centrally. Then they re-shipped the product direct to customers and distribution centers. Obviously, the cost of this double transport model was enormous.
Another U.S. based company took a different approach: they preprinted labels at their headquarters and flew them, accompanied by corporate representatives, to the manufacturing plant in Puerto Rico. Of course, every time a product changed, corporate headquarters developed the new label template, preprinted another batch of labels and flew another team to and from Puerto Rico once again.
An additional downside to this approach is that from time to time, batches of preprinted labels in remote locations may mysteriously disappear, ending up on counterfeit product lines. Also, if the old labels are not completely removed from the location, they can be mistakenly applied.
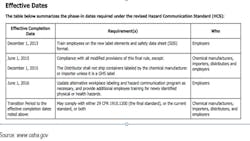
Fines for Failure to Meet Regulatory Requirements
The deadlines for OSHA’s HCS compliance in accordance with GHS are as follows:
So for chemical manufacturers doing business in the U.S., there are the looming OSHA HCS phase-in deadlines. With the emergence of global standards, additional Safety Data Sheets (SDS) are required, depending on your location. For instance, there are GHS SDSs, ISO MSDSs, ANSI MSDSs, and the former OSHA MSDSs, all which need to be understood in the new vernacular and recreated with new detailed data. Having so many required documents can be cumbersome if the data are not properly managed—or centralized—so that all content can be populated correctly both on the label and on the regulated Data Sheet. It is more important than ever to be able to access data from one “source of truth” and organized with the view toward future and frequently evolving regulations.
Even before the deadlines currently in effect, a failure to comply with OSHA HCS mandates was one of the top five reasons for fines levied. The chief targets of fines are customers who accept delivery of mislabeled product. And whom will that customer blame? It is up to manufacturers to provide accurate information through effective labeling.
The complexity of labeling chemicals today is quickly apparent by reading through the U.S. OSHA “Purple Book,” a ninety-page document that explains the new labeling requirements aligning with the Globally Harmonized System. Environmental imperatives to protect human health by protecting oceans, rivers, air, soil, plants, food, animals, and fish are the underlying purpose of these regulations. Adherence is for the purpose of upholding and improving the quality of life at every level. Carefully meeting these labeling requirements shows customers and consumers that manufacturers are following best business practices, which are the basis of healthy commerce.
The fact is few chemical industry manufacturers miscalculate the time required to manufacture their product in the quantity and to the schedule demanded by a customer. These companies have invested in production, manufacturing and assembly processes, methods, and best practices that enable them to routinely meet internal manufacturing deadlines and shipping schedules. Yet some manufacturers fail to consider the potential for a labeling mishap as part of the scheduling formula.
So after a period of successfully manufacturing a product in volume, to high quality, and in keeping with exacting customer requirements, a last minute unanticipated labeling problem can derail orders. Time, money, and customers can be lost as a result. In addition to the U.S. regulations, in the global supply chain, a variety of additional standards and regulations may apply depending on the country or region. Even while meeting the most stringent standards, in addition to the trend toward the Globally Harmonized System, variations in labeling requirements and regulations can require updating and adherence for specific territories in the global market.
In today’s chemical industry supply chain, manufacturers look for value-added opportunities and improved economies of scale. Labeling inefficiencies such as redundant relabeling slow operations down, and the bottom line suffers. Scaling internally and externally creates a streamlined, cost-effective process.
3 Elements of Enterprise-Centric Labeling
1. Consolidation and Centralization
Chemical manufacturers with dispersed, departmental, standalone and multi-regional labeling systems face a daunting task of meeting enterprise-wide consistency and control. Consolidation around a centralized system, tied to enterprise applications and data, insures corporate-wide labeling consistencies, compliance, and security. Chemicals manufacturing companies need the ability to easily and quickly manage label data, make label changes, comply with evolving standards and regulations, and flexibly support new labeling requirements. Allowing multiple locations and/or suppliers access to centralized data to seamlessly produce labels remotely is crucial to business continuity. Utilizing this centralized approach to label data allows businesses to scale globally and remotely, and drives label production from any of its sites.
2. Integration
Chemical products manufacturers today know they need—and they already have—systems for version control. This means many chemicals manufacturers have systems in place for compliance and regulatory standards with approvals, workflow, revisions, and documented copies. They have the right people already in place who are familiar with these systems. With this in mind, it isn’t practical to replicate data. Instead, it makes more sense to simply use the label data in these existing applications to generate the labels; therefore, the ability to connect and integrate to all key sources of label data is essential.
Business partners, too, need to leverage their own sources of label data, and extend labels and data to their partners. Through integration, an unprecedented level of flexibility to enable the use of corporate or partner data to create, man- age, and print mission-critical barcode labels across the global supply chain becomes possible.
3. Automation Based on Business Rules
In addition to application integration, the forward-looking solution in chemicals products manufacturing labeling looks to all major enterprise applications to drive label printing. To ensure an effective global supply chain strategy, customers must consider how labeling intersects with evolving contributors such as globalization of manufacturing, safety and quality of products, shorter lead-times, lean business environments, and changing international market demands. Widely installed enterprise applications from providers such as Oracle, SAP, and others, are considered a “single source of truth,” and if these systems are leveraged to drive label printing and label data, then the error-prone practice of manual or redundant label data entry is eliminated.
Enterprise labeling solutions that provide access to sophisticated business rules logic allow customers to automatically meet the rigors of global requirements such as regulations, languages, images, formats, and printers, and to manage variability across multiple industries and regions in one place. Automating these labeling variations frees up the organization to use precious labor hours more creatively and efficiently.
Greg Wimble has more than twenty-five years experience in manufacturing and supply chain software applications. As the Chemicals Industry Specialist at Loftware, Inc. (www.loftware.com), he provides guidance to chemicals manufacturers on the implementation of enterprise labeling solutions to accomplish their goals for complex global and regulatory challenges in the global supply chain.
A free webinar, "How to Leverage GS1 Standards for Business Benefits in the Food Supply Chain," will be hosted on 10/21 and offers insights regarding most recent best practices for GS1 standards-based solutions in the food & beverage industry. Register here.