Fuel cells will be powering fork trucks in less than two years. That's what L. E. Wells, vice president of engineering at Exide, recently told a Cleveland audience. This doesn't mean fuel cells will be in general use by that time. But, said Wells, they will be advanced enough for special industrial use.
The more conservative predict military use of fuel cells in about three years and industrial use in five years.
Any way you look at it, the last lap is being run. And it may make a great difference to you as a material handling engineer.
What's the Furor?
Excitement is mounting as dozens of companies close in on the goal of a practical fuel cell. There's nothing new about the idea. Someone's been trying to develop fuel cells off and on since the idea was advanced 150 years ago. But only recently have they been able to make the idea work.
A fuel cell converts chemical energy into electrical energy without engines and electrical generators. It works opposite to the storage battery which splits the electrolyte into particles. Instead, the basic fuel cell combines hydrogen and oxygen to form water, releasing electrons which flow as electricity. You release more energy when forming water than when decomposing it.
What's it Good For?
Dr. Karl Kordesch of Union Carbide Corp. puts it like this:
"There is no advantage in using fuel cells where high power output per unit weight is required for only a short time. For this high rate primary batteries are certainly superior. Also, there is no indication that fuel cell power will compete with network power—at least in the near future.
"However, the picture changes if you want, for instance, a 100-watt power source where you can't use a motor-generator because of interference, noise, fumes, etc. A 50-lb conventional storage battery can supply this power for 10 hours. Over a month, this means 72 battery exchanges, or moving 3,400 lb.
"A hydrogen fuel cell delivering 100 watts should weigh about 50 lb. This includes a hydrogen generator. From less than 0.6 lb of lithium hydride (source of hydrogen), you can produce 100 watts for 10 hours. Total fuel weight for the month then comes to only 43 pounds.
Some Early Applications
The Hydrox fuel cell powered a 4,000-lb-capacity fork lift truck in England.
• Allis-Chalmers ran a 5,270-lb tractor with 1,008 fuel cells packed into the engine space. Drawbar pull measured 3,000 lb.
• Exide powered a small racing car with a fuel cell. The firm also has signed agreements with 12 fork truck makers to help with applications and design.
• National Carbon Co. developed fuel cells, which have been running Army portable radar sets for more than a year.
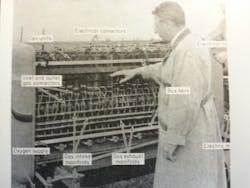
FIFTEEN KW from a fuel cell gives this tractor 3,000 lb of drawbar pull. There are 1,008 individual, cells. Researcher T. G. Kirkland points to a 9-cell unit. Electricity generates from a mixture of fuel gases, largely propane, and oxygen in an electrolyte.
Other companies have fuel cells undergoing rigorous tests in their laboratories. The day of reckoning is fast approaching.
A Peek Farther Ahead
Fuel cells may be fine for automobiles. They have all the advantages and few, if any, of the disadvantages of batteries in the old electric cars. Fuel cells take large overloads without damage. And lack of moving parts makes them silent and vibration-free.
Applied to stationary power, fuel cells could use off-peak power. Excess energy could break water into hydrogen and oxygen, which would be stored until needed again for fuel cells.
Atomic reactors used to create power could be kept on the job during off-peak hours to produce gas for fuel cells. Reactors work more efficiently at full capacity.
Why Are Fuel Cell. Good?
Above all, fuel cells are efficient. Test models run at 60% to 80% efficiency. Developers predict efficiencies near 100 percent. The best gas turbines are 40% efficient. Internal combustion engines reach 30% at peak. Good diesel engines may reach 35%.
All these systems oxidize something. The big difference is how. Turbines and engines produce heat and friction along with power. Heat and friction are lost energy. Fuel cells, on the other hand, control the heat, winding up with more useful energy.
More Fuel Cell Advantages
- Light. One type delivers 250 watt hours per pound, compared with 10 to 12 watt hours per pound for lead-acid batteries.
- Minimum maintenance. There are no moving parts.
- Flexible. They can be used singly or in series to produce a great range of power.
- Refuel or recharge quickly. Some of them go back into operation in the time it takes to fill a gas tank.
- No noise. Only the hum of the electric motor they power disturbs the golden silence.
- No smoke. Fuel cells can power vehicles that work in unventilated space
- No unpleasant wastes. The byproducts range from water to chemical compounds which often can be sold to defray some of the costs of the fuels. Some cells can recharge, producing no byproducts.
- Withstand vibration and climate extremes.
A Little History
The English got the ball rolling. Sir Humphrey Davy first mentioned fuel cells in 1801, devised one using carbon and nitric acid. Sir William Grove built a chemical battery in 1839, using the water-forming reaction of hydrogen and oxygen. Fifty years later, Ludwig Mond and Carl Langer set up a similar cell which became the basis of present day work. They obtained 6 amperes per square foot of electrode, using an acid electrolyte and platinum electrodes.
Attempts to make a practical cell continued, using hydrogen, carbon monoxide, acetylene and hydrocarbons. Around 1900, the first high temperature cell was tried.
The dramatic spurt came with a contemporary, Francis T. Bacon of England, and his Hydrox cell. It's a high temperature-high pressure cell, producing current densities up to 1,000 amperes per square foot. Another Englishman, Dr. H. H. Chambers, developed the Carbox cell, a high temperature type with a fused carbonate electrolyte.
Both of these are being further developed in the United States by Leesona Corp. of Rhode Island.
Research and development in this country is going on at a furious pace among other firms. They include Exide Industrial Division, The Electric Storage Battery Co.; Allis-Chalmers; National Carbon Co. of Union Carbide Corp.; General Electric; Pittsburgh Consolidated Coal and Coke Co., and others.
Research and development also is going on in Germany, The Netherlands, England, and, presumably, Russia. A Russian, O. K. Davytan, obtained current densities of 18.5 amperes per square foot from a high temperature cell, but there have been no further reports since 1946.
A ZINC-OXYGEN cell powers the small racer shown above, which has run for a day and a half before re-charging. The tank at the rear holds oxygen which flows at low pressure into the cell where the zinc fuel in an electrode is oxidized. The cell's electrolyte is potassium hydroxide.
Classifying Cells
You can divide fuel cells into groups. Some operate around atmospheric pressure and low temperatures (150°F). Others need pressures up to 800 psi and temperatures of 400° to 500°F. Still others operate at 1,200°F.
You also can classify fuel cells by fuel type.
There's the direct cell (using solid fuel), semidirect cell (using gaseous fuel), and indirect cell (oxidation-reduction).
WHAT KINDS OF FUEL CELLS?
Zinc Oxygen Cell
One electrode holds finely-divided zinc and the other is fed compressed oxygen. Zinc is fed by batch or continuously. Exide developed a special metal electrode, claimed superior at moderate temperatures and pressures. The electrolyte is sodium hydroxide.
The system generates zinc oxide which can be sold after it's removed. Or the zinc can be deoxidized and fed back for reuse. This type recharges like a storage battery.
Fuel Gas Cell
A mixture of fuel gases, largely propane, is adsorbed by the catalyst on the anode. It reacts with the electrolyte, generating electricity. Oxygen at the cathode reacts with an electron from the external circuit, reforming the ion used up at the anode. The byproducts are water and carbon dioxide.
To get 15 KW, Allis-Chamers used 1,008 fuel cells, each 1/4-inch thick and 12 inches square. This powered a standard 20-hp d.c. motor in a 5,270-lb tractor.
Gaibon Cell
It has chemically-treated, hollow, porous carbon electrodes, works at environmental temperatures and pressures. The cell uses hydrogen and oxygen, but can replace the oxygen with air. Evaporation gets rid of the water byproduct.
This cell is most efficient at normal usage, when no attempt is made to draw maximum power from minimum volume. The best design, according to National Carbon Co., produces about 1 KW from a unit of 1 cu ft. The ceIl is most desirable for high current, low voltage use. Increased pressure in the cell will increase electrical output.
Hydrox Cell
Hydrogen is released to one electrode and oxygen to the other. Potassium hydroxide is the electrolyte. Water is the byproduct. It operates at 800 psi and 400° to 500°F.
The hydrogen electrode is nickel-plated steel with holes drilled through it. The oxygen electrode is impregnated with lithium. Forty electrodes, each 1/16-inch-thick and 10 inches in diameter, go into a case which is clamped to withstand the pressure.
This system needs large and bulky controls, but it successfully ran a lift truck The fuel is carbonaceous, such as natural gas, kerosene, vaporized gasoline, or coal gases. Since the cells work at around 1,200°F, the electrolyte is a molten salt usually carbonates of sodium, potassium, and lithium. The fuel reacts with steam and carbon dioxide to produce hydrogen and carbon monoxide. These react with carbonate ions to form carbon dioxide and water, giving up electrons. At the positive electrode, oxygen or air takes up the electrons and reacts with carbon dioxide to produce the carbonate ions.
Sodium Amalgam Cell
Sodium dissolves in mercury to form an amalgam which reacts with oxygen from the other electrode. Water is the electrolyte. This yields sodium hydroxide. Less than 2 lb of sodium produces 1,000 ampere hours per cell. This fuel cell is capable of long term operation.
Redox Cell
Fuel and oxygen react with other chemicals rather than with each other as in the foregoing systems. Chemical intermediates generate current in the cell.
One type uses tin salts and bromine. Hydrogen adds electrons to tin ions which give up electrons to the electrode and return to react with more hydrogen.
The oxygen similarly takes electrons from bromide ions, converting them to bromine which takes up electrons from the other electrode. General Electric is developing a similar system with titanium salts.
A Hydrox fuel cell easily provides electricity to drive a lift truck in Cambridge, England. The cell, 30" x 15" x 12", produced 5 KW, about 7 hp. But control units for this cell are quite bulky.